Recently published in Clinical Cancer Research, a study led by Alena Gros, Principal Investigator of the Vall d’Hebron Institute of Oncology’s Tumor Immunology and Immunotherapy Group, tested more than 500 peptides (amino acid sequences) derived from non-canonical proteins as candidate tumor antigens that could be recognized by T-cells that activate an immune response in patients.
Cells in our body have proteins present on membranes known as HLA-I. These molecules bind peptides and present them on the cell surface. Some of these are recognized by lymphocytes or T-cells and trigger an immune response against cancer cells. One of the goals of research in immunotherapy is to seek out new tumor-specific antigens that can activate the immune response to fight cancer cells without targeting healthy tissue.
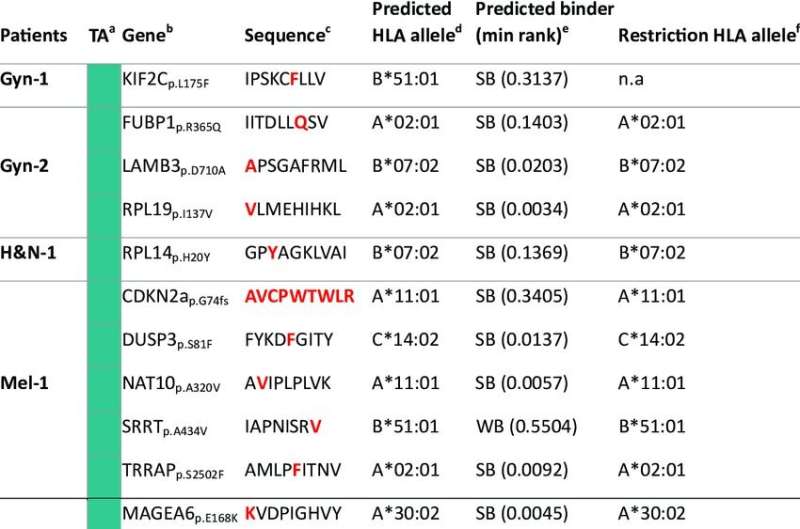
Employing immunopeptidomics, the investigators identified peptides presented on HLA-I in patient-derived tumor cell lines of melanoma, gynecological, and head and neck cancers. They discovered more than 500 peptides derived from non-canonical proteins as candidate tumor antigens.
To determine the role of non-canonical peptides in immune surveillance the researchers evaluated the presence of spontaneous pre-existing T-cell responses in cancer patients, targeting non-canonical tumor antigens as well as other conventional candidates. They observed that a high percentage of the conventional candidate antigens were recognized by the patients’ T-cells but found no T-cell receptor antigen recognition of the 507 non-canonical candidates.
“This finding is important. Up until now, research into non-canonical proteins using bioinformatic tools has suggested that we would be able to identify a new source of targetable tumor antigens to spontaneously activate an antitumor response in patients. Our present results have subsequently put this line of research on hold for the moment,” explains corresponding author Alena Gros.
“Despite these peptides did not trigger a T-cell response in cancer patients, we sought to establish if it was possible to select T-cells with receptors that specifically recognize these peptides in the laboratory, which would enable us to exploit these as immunotherapy in patients,” says Maria Lozano-Rabella, a Postdoctoral Fellow of Alena Gros’ Group and first author of this present study.
“As one door closes, another opens. Our present results describe three T-cell receptors specific to three non-canonical HLA-I tumor ligands as novel antigens and potential therapeutic targets,” observes Gros.
Upon selecting and amplifying T-cell populations with these receptors in the laboratory, the investigators assessed the pattern of tumor specificity of the three candidate antigens to ensure that they recognize tumor cells but did not elicit an immune response against healthy cells. To do so, they co-cultured selected T-cell populations with patient-derived tumor cell lines and healthy cell lines.
“We observed that one of the candidate non-canonical peptides elicited a T-cell response to various tumor cell lines, but did not target normal cells. As such, we will continue to investigate its potential use as a therapeutic target in cancer patients,” adds Maria Lozano-Rabella.
Results demonstrate that there are regions of the genome, previously considered as non-coding, that in fact are. The investigators have evidenced there are non-canonical HLA-presented proteins shared by various types of tumors with a promising pattern of tumor specificity, and identified specific T-cell receptors for these peptides.
“While our findings suggest a limited contribution of non-canonical proteins to cancer immunosurveillance, they represent a first step toward exploiting their potential in the development of immune-based strategies in oncology,” concludes Alena Gros.
Vall d’Hebron Institute of Oncology

