Patients suffering from chronic liver disease don’t respond to vaccination and are at high risk of viral infections. In these patients, virus-specific T-cells are defect and unable to eliminate viral pathogens. A research team led by ImmunoSensation2 member Prof. Zeinab Abdullah at the University Hospital Bonn (UKB), in collaboration with colleagues from the University of Oxford and the Technical University Munich, has now discovered the molecular mechanism underlying the suppression of T-cell immunity.
The researchers could show that targeted inhibition of a single immune receptor can reconstitute the immune responses to vaccination against Hepatitis B and COVID-19 in patients with chronic liver disease. The results are now published in the Journal of Hepatology.
Chronic liver diseases (CLD), such as liver cirrhosis and fibrosis, have various implications apart from direct liver functions: Patients have an enhanced susceptibility to viral infections, which are often intractable and may result in life-threatening disease. Also, CLD patients show weak responses to vaccination.
Both could be due to an impaired functionality of the acquired, technically called adaptive, immune system. A certain type of immune cells, the T-cells, is responsible for the generation of immunological memory after an infection or vaccination. Loss of T cell immunity in patients with chronic liver disease is a recognized clinical complication. In order to reconstitute functionality of the immune system in CLD patients, it is important to understanding the mechanisms underlying the loss of T cell immunity during liver injury.
Gut bacteria in the liver
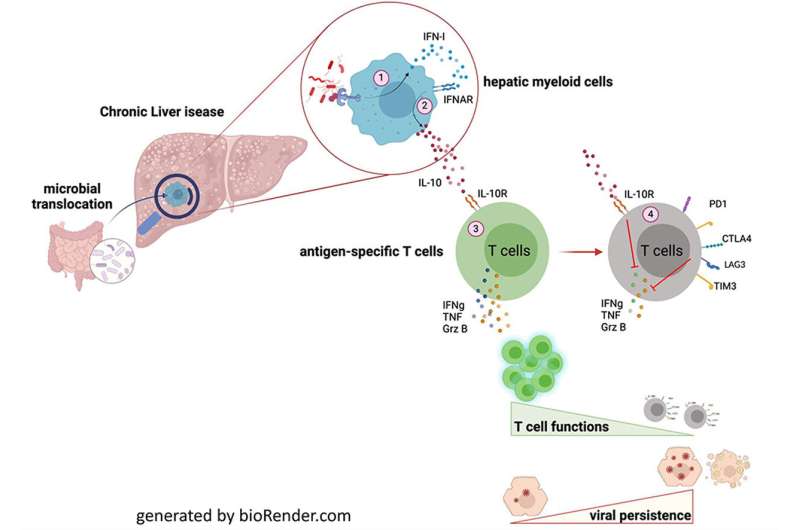
Chronic liver diseases are often accompanied by a pathological change of the intestinal flora. This weakens the barriers of the gut, allows bacteria to enter the circulation and thereby ultimately the liver. The translocated microbiota activate the immune cells in the liver and induce the release of type I Interferone (IFN-I). This endogenous messenger molecules, acts as an alarm signal for surrounding immune cells in the liver.
“The alarmed innate immune cells in turn release another anti-inflammatory cytokine called Interleukin 10,” explains Prof. Zeinab Abdullah from the Institute for Molecular Medicine and Experimental Immunology at the UKB. “We identified Interleukin 10 as the key mediator of impaired T-cell functionality in CDL.”
“When we looked at the virus-specific T-cells in the context of chronic liver disease we observed identical gene signature as in exhausted, dysfunctional T-cells in cancer and chronic viral infections,” says Dr. Susanne V. Schmidt, from the Institute of Innate Immunity at the UKB. A hallmark of these defect T-cells was the upregulation of genes induced by the anti-inflammatory cytokine Interleukin 10 (IL-10).
These T cells are no longer able to do their job as part of the immune response, resulting in impeded virus clearance and a diminished response to vaccination.
Blocking of immune signaling rescues T-cell function
The scientists were able to show, that a specific neutralization of IFN-I and IL-10 leads to reconstitution of T cell immunity against viral infection. “We identified the IL-10 signaling pathway as a potential molecular target to restore the T-cell mediated immune response and also the vaccination efficacy in CLD patients” Abdullah states.
In order to detect IL-10 in their surrounding, T-cells make use of an IL-10 receptor on their surface. By specifically blocking this receptor, the scientists were able to restore anti-viral immunity in mice. In a subsequent clinical study, the finding was approved for T-cells taken from vaccinated cirrhosis patients. Also, the treatment did not show any immune-pathological side effects, rendering IL-10 signaling a promising target to reconstitute T-cell immunity in CLD patients that can be further explored in future clinical studies.
“Our study highlights the fundamental role of microbiota and the gut-liver axis in the suppression of antiviral immune functions. Not only in patients suffering from CDL but also for the systemic persistence of viral infections and poor responsiveness to vaccination” Abdulah closes.
Inka Väth, University of Bonn

