Type 2 diabetes is a chronic disease in which the body does not produce enough insulin, or does not use it efficiently. It is caused by the combination of a genetic predisposition to obesity, sedentarism and an unhealthy diet, and it affects millions of people around the world. Now, researchers of the University of Barcelona (UB), the Institute for Research in Biomedicine (IRB) and the Diabetes and Associated Metabolic Diseases Networking Biomedical Centre (CIBERDEM), have identified a molecular mechanism involved in the development of this disease.
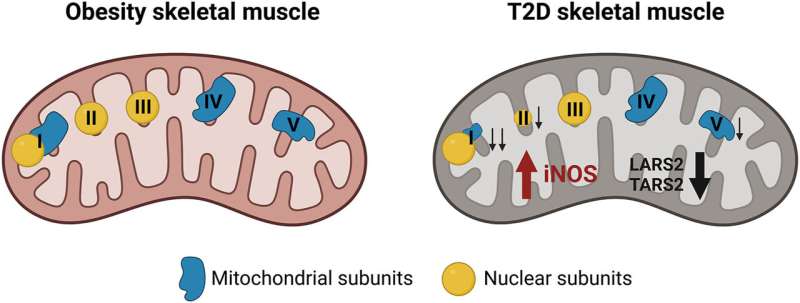
The study, published in the journal Redox Biology, has described—in patients and animal model samples of type 2 diabetes—a decrease in mitochondrial proteins that synthesize complex subunits of the respiratory chain. This decrease in proteins is associated with an increase in intracellular nitric oxide which, according to the researchers, could be a method for diagnosing the disease.
Mitochondria are the organelles that produce cell energy, and there is evidence relating dysfunctions in its functioning with insulin resistance, typical of type 2 diabetes. The aim of the study was to determine whether there were alterations in the complex subunits of the mitochondrial respiratory chain that could be associated with this mitochondrial dysfunction.
Then, the researchers wanted to explore whether nitric oxide—a present molecule in mitochondria that acts as a cell messenger in several physiological and pathological processes—is involved in these alterations.
To do so, the researchers analyzed muscular samples of obese patients with type 2 diabetes (it usually appears around the age of 55), obese patients with early diabetes (around the age of 25), and samples of model animals with diabetes.
“In this study, conducted in collaboration with clinical doctors from Dublin City University and the Trinity College Dublin’s St James’s Hospital (Ireland) and researchers from IRB Barcelona, we found that mtRNA synthetases (proteins that synthetize the mitochondrial complexes) play a relevant role in the defects observed in mitochondrial respiration, since its decrease involves the decrease in synthesis of specific subunits of the respiratory chain complexes and, therefore, a mitochondrial dysfunction associated with a larger production of reactive oxygen species (ROS), and specifically, nitric oxide,” notes Maribel Hernández-Alvarez, researcher at the Faculty of Biology of the UB, the Institute of Biomedicine of the UB (IBUB) and CIBERDEM, who led the study together with Antonio Zorzano (UB-IRB-CIBERDEM).
These results open the door to more research into the effects of nitric oxide-producing enzymes and how they affect the abundance of mtRNA synthetases and their relationship to mitochondrial protein synthesis.
University of Barcelona

