Experiments at 48 elderly care facilities in China find that salt substitutes lowered diastolic blood pressure and resulted in fewer cardiovascular events, according to a paper titled “Salt substitution and salt-supply restriction for lowering blood pressure in elderly care facilities: a cluster-randomized trial,” published in Nature Medicine. Rachael M. McLean has published a News & Views piece in the same journal discussing the findings.
Researchers on the Peking University-led study conducted clinical trials with 48 residential elderly care facilities involving 1,612 subjects, including 1,230 men and 382 women. Subjects were cluster-randomized and had meals containing either a salt substitute (62.5% NaCl and 25% KCl), regular salt or a progressively restricted salt for two years.
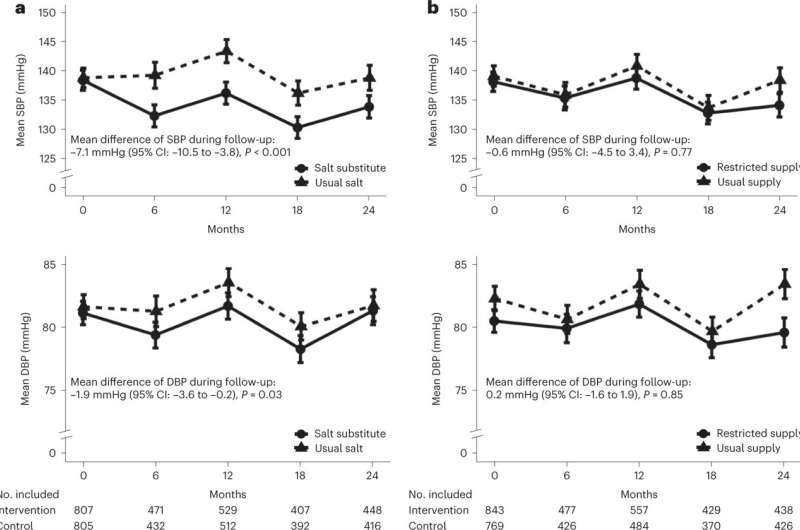
According to the study authors, salt substitute, compared to salt, lowered systolic blood pressure by 7.1 mmHg. In reference to this study, salt intake averaged 3,749 mg per day, nearly double the World Health Organization’s suggested target of less than 2,000 mg of sodium per day. One reason the researchers chose the facilities was based on the tendency of the regions to have higher dietary sodium intake.
The restricted salt supply was compared with the usual amount of salt and salt substitute and showed no effect on systolic blood pressure. The salt substitute diet also lowered diastolic blood pressure by 1.9 mmHg. It also resulted in fewer cardiovascular events but had no effect on total mortality. However, reduced salt was associated with a higher mortality rate, according to data provided in the study.
Salt substitute increased mean serum potassium and led to more frequent biochemical hyperkalemia but was not associated with adverse clinical outcomes. In contrast, the study states that salt restriction had no effect on any outcome, in contrast to the data provided.
Safety was assessed among 1,086 subjects with blood assays during follow-up. Subjects without follow-up assays were more likely to be older, women, better educated, bedridden or severely ill. The authors state that there was no difference in baseline characteristics between randomized groups in those with and without follow-up blood assays and provide no further details about which meal conditions these subjects were subject to.
Despite reported results, one aspect of the experiment failed. Researchers had hoped to compare the salt substitute with a cohort that received progressively reduced salt in meals with a goal of about 40% reduction. The study design relied on the meal preparers and facility managers to oversee this aspect of the study and suggests that kitchen staff may have been resistant to the intrusion of the usual meal prep.
Additionally, analysis of self-reported data indicated that subjects were able to detect the reduction in salt use and some added table salt to their meals. This also creates the possibility that meal prep staff who failed to reduce salt were responding to feedback from diner complaints about their cooking. One potential reason for this noncompliance could be that the progressive salt reduction strategy attempted to reduce the salt of meals without drawing notice, which could extend to not informing the subjects.
If the participant were unaware that they were part of a salt reduction study or unwilling to participate within the study parameters, it could raise ethical questions. The minimum requirements for consent to be informed in human research are that the participant understands what the research is and what they are consenting to. Statements in the paper such as “…subjects were able to detect the reduction…” suggest they may have been unaware that their food was part of an experiment in which they were included.
Whether this consent should be extended to a cohort that is receiving an alteration of diet that could otherwise be considered healthy may not matter, and it is unclear if the study subjects were informed about the salt reduction strategy based on the universal non-compliance by subjects as well as facility staff.
Then again, it could be that salt reduction subjects, who had a median age of 72.2 (the oldest age cohort in the study), fully consented initially and later decided that life was too short to eat bland food.
The paper states, “The study was approved by the Peking University Institutional Review Board with a group consent obtained through discussions between the local study investigators, the administrator of each facility and the government agencies responsible for the facilities. All subjects that did the baseline and follow-up surveys provided written informed consent.”
The results of this trial indicate that salt substitutes may be an effective way to lower blood pressure and reduce cardiovascular events in the elderly.
The study implementation makes it impossible to say what the comparative effect salt reduction alone would have on a similar population. While fully acknowledging it took place, it is unclear how the researchers accounted for the non-compliance yet still managed to include data and statements about the salt reduction in the study.
Justin Jackson , Medical Xpress

