Since the introduction of SARS-CoV-2 mRNA vaccines, there has been an ongoing debate about whether vaccinations or natural immunity provide superior protection. New research shows that while both build immunity to the virus, mRNA vaccines side-step the development of self-attacking antibodies—known as autoantibodies—that frequently occur in COVID-19 patients.
Evidence increasingly supports a connection between COVID-19 infection and autoimmunity, in which patients’ immune systems target their own tissues. However, it was unclear if vaccination posed the same risk. To investigate, a team led by Aaron Ring, MD, Ph.D., associate professor of immunobiology; and Akiko Iwasaki, Ph.D., Sterling Professor of Immunobiology and professor of dermatology; of molecular, cellular & developmental biology; and of epidemiology (microbial diseases), measured the presence of self-reactive autoantibodies in blood samples from individuals before and after vaccination and compared this to changes in autoantibody levels in COVID-19 patients.
They found that while many new autoantibodies formed in infected patients, they did not see new autoantibodies in those who received the vaccination. Their findings were published in Nature Communications on March 9.
“We showed the vaccination provides all the benefit of antiviral immunity without autoantibody development,” says Ring. “So the cost you pay in terms of risk is substantially lower for getting vaccinated than becoming infected.”
“This study underscores the advantages of vaccines in generating protective immunity to the virus rather than relying on infection to do so,” says Iwasaki, who collaborated with the Ring team on this and related studies.
COVID-19 infection triggers antibody development
In 2021, a collaboration between Ring’s and Iwasaki’s labs made a major discovery that patients infected with SARS-CoV-2 develop diverse autoantibodies at a high rate. “This was a startling and extremely concerning development,” says Ring.
Intrigued, the duo turned their attention towards vaccination. They had several reasons to wonder if the mRNA vaccines posed a similar risk of triggering autoantibody development. First, the vaccines themselves produce inflammation that is responsible for fever and other common side effects of the jab. More serious side effects of the vaccines also suggested the potential for autoantibodies, including rare vaccine-related adverse events, as well as flaring of existing autoimmune diseases after vaccination. Additionally, some researchers were concerned about a phenomenon known as molecular mimicry.
“The idea here is that sometimes, when the immune system mounts a response to a virus, those same antibodies can also recognize similar shapes that are found in our own biomolecules,” says Ring. “So there was concern that any immunity against COVID-19 could lead to a risk of autoimmune disease because of these off-target effects of the antibodies to the virus.”
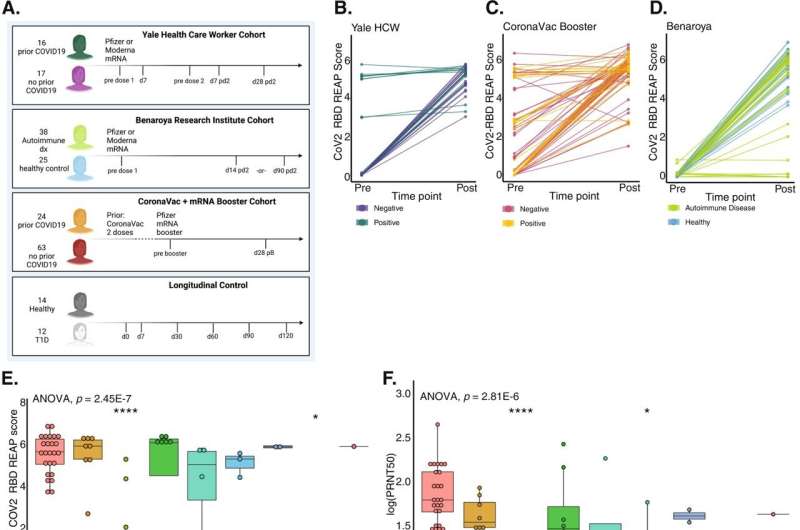
mRNA vaccine not linked to autoantibody development across three cohorts
For this latest study, Ring’s team led by MD-Ph.D. student Jillian Jaycox used an autoantibody screening technology called REAP to track the formation of autoantibodies after vaccination. “It’s like a molecular search engine for autoantibodies,” says Ring. “Within a single drop of a patient’s blood, we can see all the functional autoantibodies that are present.” Looking at a cohort of 33 healthy individuals collected at Yale, they did not find an increase in autoantibody development as they had with individuals infected with COVID-19.
Then, they joined forces with the Benaroya Research Institute in Seattle, a non-profit organization that specializes in autoimmune disease research. To test whether those with pre-existing autoimmune disease were at higher risk for developing autoantibodies post-vaccination, they studied a cohort recruited by Benaroya consisting of 38 individuals with an autoimmune condition and 25 healthy controls. Once again, neither group showed heightened autoantibody development.
Finally, with the help of collaborators in the Dominican Republic, the team studied a third cohort of 87 volunteers who received the mRNA vaccine as a booster after originally receiving the inactivated whole viron CoronaVac vaccine. As with the prior cohorts, they didn’t see a rise in autoantibodies.
During the study, the investigators also studied autoantibodies in the blood of individuals who weren’t vaccinated. They found that there was some natural fluctuation in autoantibody levels, but there was no significant difference between the natural fluctuation and vaccination. “This gave us a lot of confidence that if autoantibodies do arise in some patients after vaccination, it must be rare,” says Ring. “And certainly, compared with infection, there is a drastic difference.”
Vaccination offers greater benefits than natural immunity
In February 2023, a report came out that found that natural immunity provided protection from the virus at least as good as immunization, spurring debate about which was better. Ring says the new Yale study swings the pendulum back toward vaccination. “We showed that with vaccination, you get all the benefits of antiviral immunity without the autoantibody development,” he explains.
Ring and Iwasaki have a long-standing and extensive collaboration. Beyond the current study, the investigators have also been working together to examine the role of autoantibodies in long COVID as well as in rare vaccine-related adverse events under the Yale LISTEN study. In addition, they are interested in autoimmunity following infections beyond COVID-19 such as influenza and other respiratory illnesses.
Isabella Backman, Yale University

