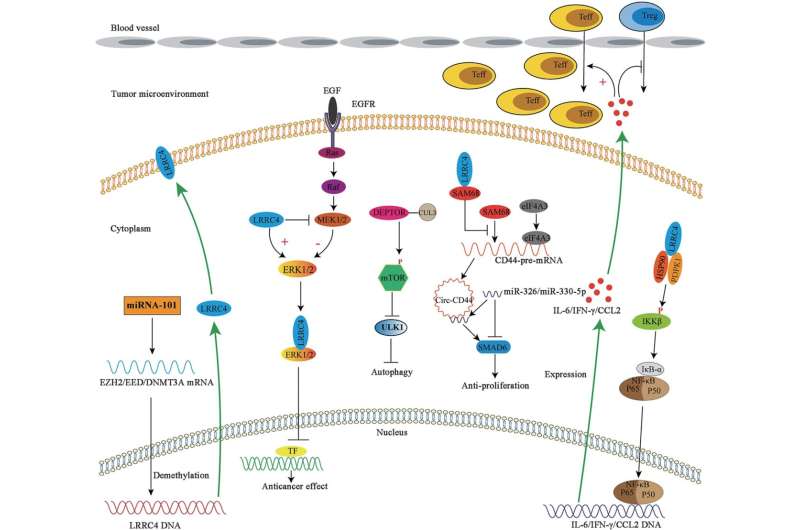
Leucine-rich repeats containing 4 (LRRC4)—a gene abundantly found in the brain and located on human chromosome 7q31–32—plays a pivotal role in memory formation, autism, spinal cord injury, as well as in determining the malignant potential, development, and progression of glioblastoma (GB), an aggressive cancer involving the brain and/or spinal cord.
In a review article published in the Chinese Medical Journal, researchers shed light on the key physiological mechanisms regulated by this gene, and the effects of its mutation.
Senior author Dr. Minghua Wu from Central South University, China, says, “In this review, we mainly discuss the effects of LRRC4 on memory by promoting the formation of excitatory synapses and long-term potentiation (LTP), the effects of LRRC4 mutation on psychoneurosis, and the new mechanism of LRRC4 in GB genesis and progression.”
Our brain functions in a complex way, with each region performing a specific role. The hippocampus, for example, is the memory center of the brain—it coordinates learning and memory performance. After receiving information from the cerebral cortex, the hippocampus processes information, then forms a memory, and stores it with the help of neuronal synapses.
Quite interestingly, LRRC4—also known by the name “netrin-G ligand 2 (or NGL2)”—has been shown to interact with the protein netrinG2, which controls the formation of neuronal circuits and synapses, even in the brain. LRRC4/NGL2 is highly expressed in multiple key brain regions and also supports memory formation in the hippocampus. As a consequence, the deletion of the LRRC4 gene adversely affects synaptic plasticity (i.e., the ability of neuronal synapses to remodel as necessary) and memory formation in the hippocampus.
Experiments conducted on laboratory mice reveal interesting facts. For instance, LRRC4 is not present during the early (embryonic) development of the mouse brain. However, subsequent increase in the expression of LRRC4 is associated with the formation of neuronal synapses. This activity is mediated by mouse embryonic hippocampal neurons in conjunction with LRRC4. The aforementioned animal model-based experiment bears testimony to the fact that LRRC4 plays a key role in learning and memory formation.
This gene also seems to play an important role in nerve fiber (or axon)-mediated vision in mice. The removal of LRRC4 from the retinal horizontal cells or neurons of developing or mature mice results in abnormal axonal growth and synaptic reduction. Neuronal function is restored when LRRC4 is re-introduced.
Research also suggests that LRRC4 promotes presynaptic and postsynaptic connections. It also encourages the accumulation of the neuronal protein N-methyl-D-aspartate receptor or “NMDAR,” which results in the formation of excitatory synapses that play a pivotal role in neurotransmission. Once again, this observation underscores the importance of LRRC4 in the neuronal circuitry.
The synergy between LRRC4 and NMDAR has major biological implications. Long-term potentiation (LTP)—a process that involves the strengthening of neuronal synapses and boosting of signal transmission between neurons—gets a helping hand from LRRC4, as evidenced by laboratory experiments. Mice lacking the LRRC4 gene show LTP inhibition. However, because NMDAR serves as a trigger for the induction of LTP, this inhibition can be easily reversed by activating NMDAR with a pharmaceutical drug.
LRRC4 also seems to play a significant role in various pathologies. Lead author of this review, Dr. Kun Deng, who also conducts research at Central South University, China, adds, “The aberrance of LRRC4 is thought to be associated to the occurrence of autism and spinal cord injury.”
Prior studies also suggest that LRRC4 mitigates clinical symptoms in mouse models of multiple sclerosis, an autoimmune disease targeting neurons, and protects neurons from immune system-induced damage. Lastly, LRRC4 inhibits a key survival process in GB cells, thus demonstrating its therapeutic potential. Temozolomide (TMZ) administration constitutes the initial treatment for patients with GB.
Dr. Wu says, “Combining the re-expression of LRRC4 and TMZ treatment prolonged the survival of mice with tumor xenografts, indicating the potential of LRRC4 as a prognostic marker for TMZ sensitivity in patients with GB.”
In summary, LRRC4 helps regulate synapse formation, stability, and excitatory transmission, and is involved in brain development, learning, and memory formation and storage. It also has key implications in neuronal disorders and aggressive brain and spinal cord cancer. Upregulation of this gene’s expression could prove to be a valuable strategy to treat mental and neuronal diseases and tumors in the near future.
Cactus Communications

