A new device invented with the help of an electrophysiologist at The Ohio State University Wexner Medical Center makes a heart procedure safer for patients suffering from atrial fibrillation (AFib), a common irregular heart rhythm.
AFib affects millions of people worldwide and greatly increases their risk of stroke and heart failure. To treat AFib, doctors use cardiac ablation to help restore the heart’s rhythm. Heat or cold energy delivered through a catheter destroys the heart tissue causing rapid and irregular heartbeats.
While the procedure is effective in treating AFib, the energy from the catheter tip is used only a few millimeters from the esophagus. There is a risk that the energy can cause a rare, but often fatal, hole between the esophagus and the heart called an atrioesophageal fistula.
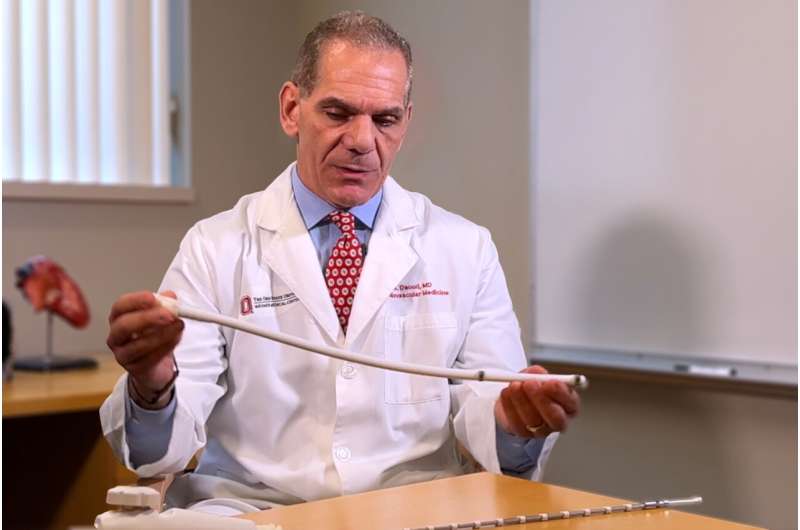
To reduce the risk of damage to the esophagus, Emile Daoud, MD, section chief of the cardiac electrophysiology program and professor of internal medicine in the College of Medicine, helped develop the concept of physically moving the esophagus away from the catheter tip during an AFib ablation procedure.
https://www.youtube.com/embed/8CG6zHR3LAo?color=whiteAtrial fibrillation, or AFib , is the most common heart rhythm problem, affecting millions of Americans and greatly increasing their risk of stroke and heart failure. For some with AFib, a catheter ablation is used to burn or freeze the precise area causing the problem to restore a normal heart rhythm. While this method is effective in treating AFib, the energy from the catheter tip can cause serious damage to the adjacent esophagus, which is only a few millimeters away. It’s an injury that can be life threatening, so an electrophysiologist at The Ohio State University Wexner Medical Center helped develop a new device that gently diverts the esophagus out of harm’s way, greatly improving safety. Credit: The Ohio State University Wexner Medical Center
Using funds from an Accelerator Award from The Ohio State University’s Keenan Center for Entrepreneurship, Daoud helped design and test the device, called ESOlution. A clinical trial in the United States and Argentina showed that using the device significantly reduced injury to the esophagus without any adverse effects. Results of the trial were presented Saturday during the Heart Rhythm Society’s annual meeting.
“It has been frustrating to not have an effective method to protect the esophagus while delivering the ablation energy at the desired location. By using suction force, we’re able to pull in the esophagus and then move the entire segment to the side by only about an inch. This creates a safe pathway to deliver the treatment,” Daoud said.
The clinical trial of 120 heart ablation patients found that without the device, over a third had esophageal injuries, but when the device was used, less than 5% had any injury to the esophagus, Daoud said. If approved by the FDA for commercial use, the device would be the first specifically developed and tested therapy to prevent ablation-related esophageal injury. Ohio State owns a piece of the technology being developed by S4 Medical Corp. Daoud is co-founder of the medical company.
“How to safely protect the esophagus has been a well-recognized problem for at least 15 years. There are several techniques such as measuring the temperature inside the esophagus and using ultrasound or CT imaging to see where it’s located, but we still have esophageal injuries. This device is effective, inexpensive and connects to a vacuum suction, which is already in every electrophysiology laboratory,” Daoud said.
Though this hasn’t been tested, Daoud believes moving the esophagus may also improve the effectiveness of the procedure. With the esophagus out of the way, doctors can safely deliver larger amounts of ablation energy when it’s needed.
Understanding where the esophagus was moved relative to the location of the ablation catheter tip may also help manage and assess patients who have concerning symptoms after ablation. Safe deviation of the esophagus means that an injury to the esophagus is unlikely the cause of a patient’s post procedure symptoms.
Amanda Mitchem, 59, of Mount Vernon, Ohio participated in the clinical trial at Ohio State and was randomly selected to have the device inserted during her ablation. She’d been suffering from AFib and simple daily tasks like doing laundry and dishes wore her out. When medication and mild electrical shocks to the heart didn’t work, she had the ablation.
“The following day was like night and day. I was breathing so much better, and I hadn’t felt that good in probably a year,” she said. “Before that, I could be just sitting and feel like I ran 10 miles.”
Now she’s back to traveling to West Virginia to play with her granddaughter and sharing her story with friends and family.
“Now I can breeze through going grocery shopping or to the flea market or yard sales. I can actually stand and have a conversation without having to gasp for breath,” she said.
Ohio State University Medical Center

