A new study by Prof. Eran Elinav’s lab at the Weizmann Institute of Science got an unexpected boost from the peace agreement between Israel and the United Arab Emirates. In the wake of this accord, Elinav was invited to a scientific conference in Dubai. On his way to the sessions, he shared a taxi with a German immunologist, Prof. Eicke Latz, and learned that unbeknownst to one another, they had been working on the same topic. “Professor Latz told me about an amazing protein complex he was studying, and I told him that my lab was studying the exact same complex,” Elinav recalls. “We began to exchange ideas in a way that helped both sides fill in the missing pieces.”
The complex that so fascinated both teams is a new kind of inflammasome—that is, one of the flower-shaped bundles of proteins that serve as the immune system’s “smoke detectors,” alerting it to infection or tissue damage. Unlike the smoke detector in your kitchen, these are assembled anew for each threat. Once they identify the danger, they trigger a massive production of inflammatory chemicals called cytokines, which then minimize tissue damage by doing away with infected or damaged cells.
When the detectors fail to do their job properly, this protective inflammatory response can’t run its proper course, and the result may be uncontrolled inflammation leading to disease. An in-depth understanding of inflammasome function could therefore help develop new therapies for a variety of inflammatory disorders.
That’s precisely why Elinav’s team embarked on the search for a new inflammasome. In fact, Elinav had a long-standing interest in the topic. During his postdoctoral research, he had identified a new inflammasome that was the first of its kind to be discovered: the NLRP6. It is mainly present in epithelial cells of the gut—the cells making up the gut lining—whereas all five inflammasomes that were known at the time function in immune cells.
Each kind of inflammasome is geared toward detecting a different type of threat, ensuring a response that is both rapid and precise. Some inflammasomes, for example, spot DNA in places where it’s not supposed to be—that is, in the cell’s cytoplasm, where it would likely be a sign of a bacterial, viral or fungal infection or a noninfectious stress to the cell.
The search for another new inflammasome began with a mystery. Inflammasomes rely on sensor proteins that belong to the same family but differ somewhat from one another, depending on the kind of threat they identify. One member of the sensing-protein family, called NLRP10, puzzled scientists because it looked like it could lie at the core of an inflammasome—except that it lacked a crucial sensing unit found in its siblings. And a smoke detector missing a sensing component can’t function. Scientists were mystified as to the roles the NLRP10 protein might play in the body.
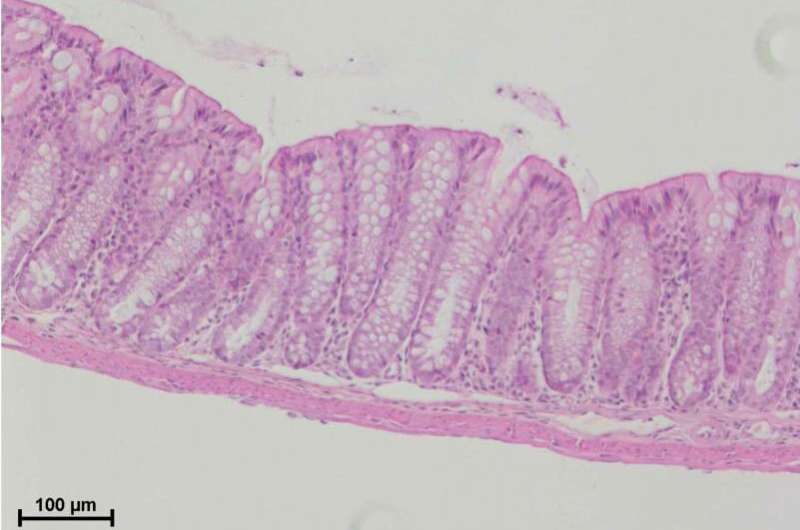
Elinav’s team, headed by Drs. Danping Zheng, Gayatree Mohapatra and Lara Kern, addressed this question by studying mice, which were found to express the NLRP10 protein in their hearts, skin and the lining of the intestine. By creating genetically engineered mice that lacked NLRP10 in the gut lining cells and comparing them to regular mice in a battery of various experiments, the researchers were able to show that NLRP10 is capable of forming a previously unknown kind of inflammasome. This suggests that despite lacking the classical sensing unit, the NLRP10 protein has other detection capabilities.
The scientists then revealed the NLRP10 inflammasome’s mechanism of action, showing that it specializes in sensing damage to the mitochondria, the cell’s power plants, which are among the first organelles to be harmed in infection or tissue injury. The moment the mitochondria are disrupted in a number of cells, the NLRP10 inflammasome picks up the danger signal, triggering a local immune response that leads to the death of these cells.
But perhaps most important, the researchers showed that the NLRP10 inflammasome might play a role in preventing inflammatory bowel disease. This disease can begin with damage to the lining of the gut caused by a self-destructive auto-inflammatory process. The gut microbes (collectively termed the microbiome) then come into contact with the normally germ-free part of the intestine, thereby triggering a potent, harmful inflammatory response. Unless immediately contained, such a response can progress to massive damage in the gut.
In the study, published in Nature Immunology, the researchers showed that mice lacking a functioning NLRP10 inflammasome in the lining of their colons were much more likely than the control mice to develop severe or even fatal inflammatory bowel disease when this lining was injured. That was apparently because without NLRP10, the first step of the healing process—containing and eliminating the initial damage—failed to kick in.
Meanwhile, Latz and his team at the University of Bonn tackled the same questions from a different direction: performing biochemical studies on skin tissue. They reached similar conclusions to those of the Weizmann researchers, showing that the NLRP10 inflammasome senses damage to the mitochondria in skin cells and suggesting that malfunctions in this newly discovered kind of sensor may play a role in inflammatory skin disease.
“This discovery adds a critical component to our understanding of the functions of epithelial cells as bona fide immune cells that provide an active and highly regulated first layer of protection against incoming threats,” Elinav says. “The next step is to find out whether the new inflammasome works in humans in the same way as in mice. We might then be able to create a drug that would activate it, so as to speed healing or prevent the damage to tissues that occurs in inflammatory diseases of skin or the intestines.”
Weizmann Institute of Science

