Rare diseases are often caused by defects in genetic material. If children inherit a defective gene from only one parent, they often are asymptomatic “carriers”—or at least that was the previous assumption. However, a research team from the University of Basel and the University Hospital Basel is now reporting that such carriers can also suffer from life-threatening diseases—and that rare hereditary diseases are therefore probably more common than previously thought.
Every child receives one set of chromosomes from their mother and one from their father. So for the majority of all genes, every human being has two copies—known as “alleles.” A lot of rare hereditary diseases only emerge if both alleles of a gene carry a defect. This is also referred to as a “recessive” hereditary disease. If only one allele is affected, the other can compensate and no symptoms will occur.
Recessive hereditary diseases include a large number of immunological disorders that are based on mutations in one of the estimated 2,500 to 5,000 genes that are relevant to the immune system. These diseases are characterized by susceptibility to infection or autoimmunity, in which the body launches an immune attack against itself.
Researchers led by Professor Mike Recher from the University of Basel and University Hospital Basel are now using the example of a recessive hereditary disease to show that the defect poses the risk of a restriction in immune system function even when it is present in only one allele.
“These kinds of cases have been far too frequently ignored in the past, based on the assumption that defects are only problematic if they are present in both alleles,” Recher explains. “However, carriers can actually suffer from life-threatening diseases too, often as adults and with symptoms that can sometimes be uncommon.”
The study, which also involved researchers led by Dr. Hiroyuki Yamamoto from the National Institute of Infectious Diseases (NIID) in Tokyo, Japan, has been published in the Journal of Allergy and Clinical Immunology.
Not enough enzyme for full function
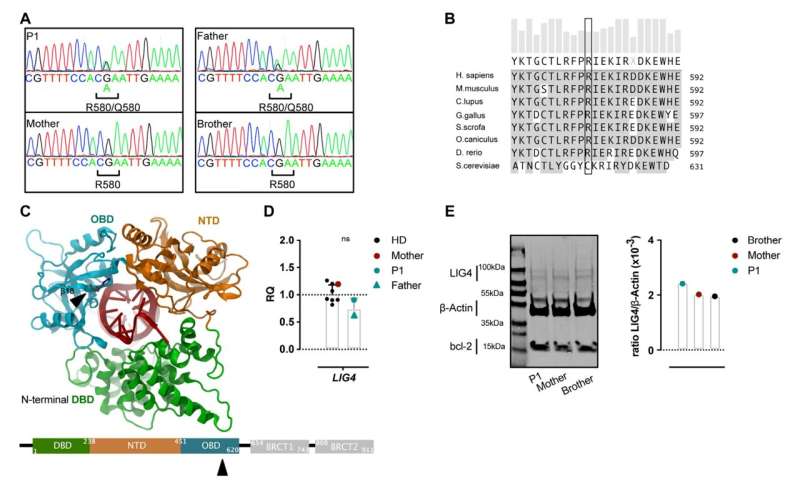
In the study, the researchers report on mutations in the blueprint for an enzyme that is crucial for the diversity of antibodies and T-cells. Mutations in both alleles of this LIG4 gene lead to a major disruption of the body’s immune response, and therefore to an increased risk of severe infections from an early age.
Previously, carriers of just one defective LIG4 allele were considered asymptomatic. But Recher and his team at the Department of Biomedicine are now reporting multiple cases where individuals have nevertheless exhibited severe symptoms that are only partially reminiscent of the original inherited disease. “In the case of these individuals, only having one functioning LIG4 gene does not seem to be sufficient,” says the immunologist.
Unrecognized risks
Among the thousands of genes that are involved in the human immune system, there are a lot of mutations in just one allele where not enough is yet known about their importance for an effective immune response over the course of a person’s lifetime. “Our and other recent findings are showing that these defects may be the cause of previously unexplained immune disorders much more frequently than previously thought.”
“We suspect that many rare recessive diseases may actually have more common counterparts, partly yet to be described, associated with unusual symptoms, a tendency to occur later in life and with a different inheritance pattern,” Recher explains. But there will continue to be healthy carriers. “In addition to genetics, environmental factors such as infections or epigenetics also play a role here.”
According to Recher, it is important that these new findings be taken into account in diagnostics. “When you understand what the problem is at a molecular level, this can suddenly open up some very targeted treatment options that are often low in side effects, and that don’t just fight the symptoms but also the cause.”
University of Basel

