Chemogenetics is a fairly new area of neuroscience that explores the use of synthetically derived receptors and selective ligands to temporarily activate or deactivate specific brain areas. These receptors are also known as DREADDs (Designer Receptors Exclusively Activated by Designer Drugs). DREADDs are now widely used in neuroscience and biology to modify neural activity and behavior temporarily.
So far, the chemogenetic actuator that was most commonly used in DREADD implementations is clozapine-N-oxide (CNO). While it has sometimes proved valuable in neuroscience studies, this actuator has a number of drawbacks and limitations, including a slow action and possible side effects associated with its back-metabolism into clozapine, a different actuator that has been found to have extensive off-target effects.
With this in mind, researchers at the National Institute of Radiological Sciences in Japan and University of North Carolina at Chapel Hill have recently introduced deschloroclozapine (DCZ), a new chemogenetic actuator that acts rapidly and is selective, high-affinity, metabolically stable and brain-penetrable. This new actuator, presented in a paper published in Nature Neuroscience, could eventually be used to rapidly induce reversible changes in the working memory and behavior of animals and potentially even humans.
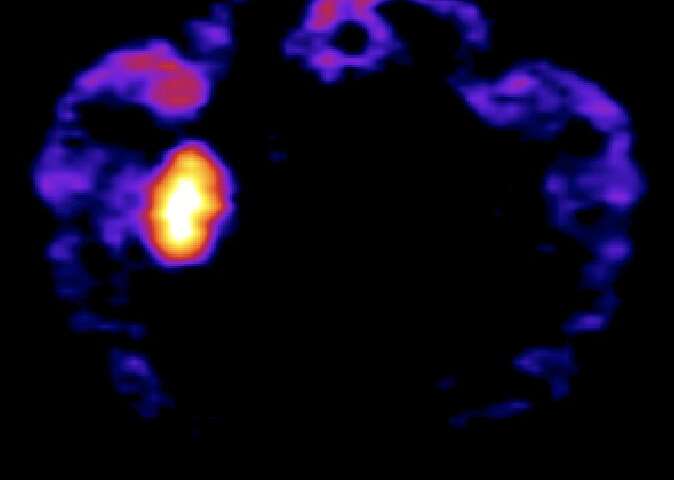
“We investigated the selectivity as well as the rapidness of the DCZ action,” Takafumi Minamimoto, one of the researchers who led the study, told MedicalXpress. “We first injected radiolabelled DCZ to animals expressing DREADDs locally within the brain and scanned them with positron emission tomography (PET).”
Minamimoto and his colleagues tested the chemogenetic actuator they designed on both mice and monkeys. They recorded the neural activity of the animals after DCZ injections using two techniques: two-photon calcium imaging for mice and electrophysiology for monkeys.
The injected DCZ rapidly penetrated into the brain of the animals, selectively binding to and occupying DREADDs. In addition, the researchers found that the systemic injection of very low doses of DCZ increased the activity of neurons expressing excitatory DREADDs within minutes, which was never achieved using previously designed chemogenetic actuators, such as CNO.
“The excitability induced by DCZ was much higher than those induced by a 100-fold high dose of previous DREADD actuators such as CNO,” Minamimoto said. “We also showed behavioral modification in monkeys with DCZ. The monkeys expressing inhibitory DREADDs in the prefrontal cortex [exhibited] impaired working memory every time after DCZ injection. Importantly, we did not find off-target action, meaning no neuronal/behavioral modulation was found for tissues/animals without DREADD receptor.”
The recent study carried out by Minamimoto and his colleagues could have a wide range of implications for chemogenetics applications. In fact, the chemogenetic actuator they designed overcomes most of the limitations of CNO, acting rapidly, selectively and effectively, without off-target effects.
“We believe that all DREADDs users will receive great benefit from DCZ with increased reliability, that is, by removing concerns about potential off-target responses,” Minamimoto said. “Together with the ability of non-invasive monitoring of DREADDs expression using PET imaging, DCZ provides a superior platform for rapid and reliable neuronal modulation, linking the causal role of neural circuits to cognitive and behavioral outcomes.”
Due to its reliability and effectiveness, DCZ could soon become widely used to conduct reversible chemogenetic interventions. In addition to being highly reliable and effective, the new actuator has great translational potential, which means that it could have a wide range of possible uses. For instance, it could be used to control the neuronal activity of patients suffering from epilepsy with minimally invasive procedures.
In their next studies, Minamimoto and his colleagues would like to try using the DREADD/DCZ system explored in their recent paper to modify specific brain circuits and genetically defined neuronal populations in monkeys.
“We plan to fine-tune the DREADD/DCZ system for more specific modulation of brain function in monkeys,” Minamimoto said. “For example, visualization of axon terminal DREADD expression combined with local drug application will allow for control of a specific neural circuit. Another example is cell-type specific (e.g., glutamatergic, dopaminergic or etc.) expression and control by the DREADD system.”
Ingrid Fadelli , Medical Xpress

