Imperial researchers have created a traumatic brain injury (TBI) computer model that maps blood vessels in a rat brain in the highest resolution yet.
They say the models could help improve our understanding of how blood vessels are affected by TBI, as well as its effects on the protective layer encasing them known as the blood-brain barrier (BBB), which protects the brain from harmful circulating molecules and pathogens.
If the methods translate well onto human brains, they could help improve our understanding of how TBIs develop and how best to treat and protect against them. The simulations could even help to replace animal models of TBI, potentially reducing the use of animals in brain research.
TBIs are the commonest cause of chronic disability in under 40-year-olds and result from severe blows or jolts to the head—commonly during road traffic incidents, falls, and assaults. Symptoms can include headaches, dizziness, fatigue, irritability and memory impairment.
Beginning at the site of impact, mechanical forces travel in waves through the brain, twisting, stretching, and shearing brain structures as the injury cascades. These forces are known to affect blood vessels, but the finer details of the relationship between mechanical forces and vascular injury are yet to be pinned down.
Now, researchers at Imperial College London have created a computer model of TBI which maps the network of vessels in the brain—called the vasculature—in the highest resolution yet, incorporating rat brain vessels just 10 microns in diameter.
https://www.youtube.com/embed/VZ2RheUku2A?color=whiteCredit: College London/Shanghai Institute of Materia Medica
Using the models, they found that adjacent blood vessels sustain profoundly different levels of stress depending on their alignment with neighboring ones. Blood vessels at 90 ° angles to others were less likely to be damaged, and vessels could be stretched to up to 14 percent of their original length without injury, while stretching by more than this amount would result in injury.
Lead author Dr. Siamak Khosroshahi, who conducted the work while at Imperial’s Dyson School of Design Engineering said: “Our unique approach explains the unrecognized role of the vascular anatomy and shear stresses in how large forces cascade through the brain. This new understanding could contribute to improving TBI diagnosis and prevention.”
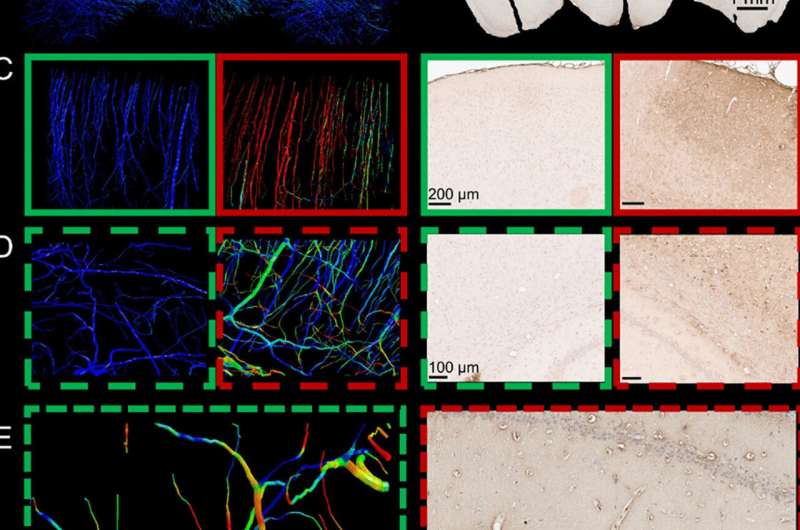
The degree to which the BBB lets molecules into the brain is known as permeability. The barrier can become more permeable after injury, making it more likely to let pro-inflammatory molecules reach the brain and usher in further injury.
Co-author Dr. Magdalena Sastre of the Department of Brain Sciences said: “Investigating vascular damage in head injury is important because a damaged blood-brain barrier can let harmful molecules in that worsen the initial injury.”
By using rat models of TBI, the authors demonstrated that greater BBB permeability occurs in TBI as a result of disruption of the vasculature, and that this is most evident soon after injury.
From this information they created brain models digitally in high enough resolution to highlight the vasculature. They found that the computer models allowed them to accurately predict the distribution of stress in the small blood vessels of the rat brains. The models also allowed them to slow down time to look at the details of TBI more closely.
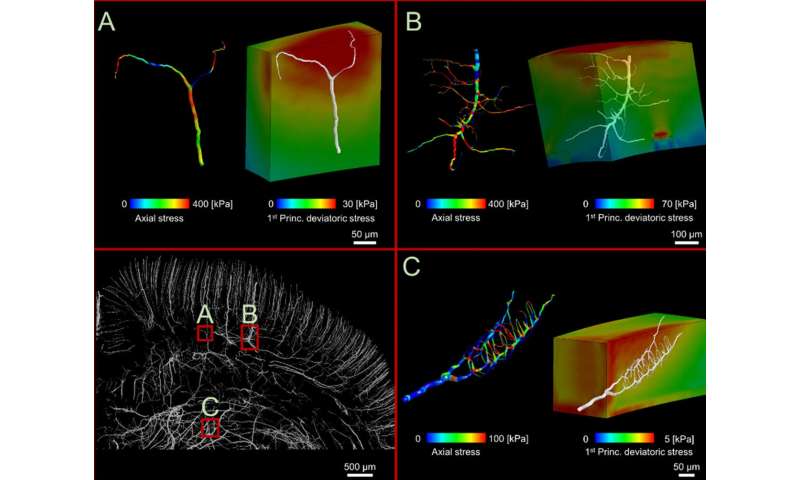
Senior author Dr. Mazdak Ghajari, also of Imperial’s Dyson School of Design Engineering, said: “Injury happens in a fraction of a second, making it hard to observe exactly what goes on. By slowing down the process, we can pinpoint exactly which brain areas sustain the most damage and go some way to understanding why.”
Co-author Professor David Sharp, also of the Department of Brain Sciences said: “This exciting new model provides insights into the way head injuries lead to brain hemorrhage. Our major trauma centers are geared up for rapidly managing bleeding within the skull as this can be life threatening. However, we don’t understand how head injuries produce different types of bleeds, which limits our ability to predict what kinds of head injury are likely to lead to hemorrhage. The development of this model is an important step in understanding this important process.”
The new, high resolution computer simulations could provide a blueprint for studying TBIs using more computers and fewer animal models, in line with the principles of Replacement, Reduction and Refinement (the 3Rs) in animal research.
The researchers say their models could also provide a more objective way to assess protection systems like helmets. Future studies on humans that include detailed reconstructions of the biomechanics of TBI are also needed to confirm the findings before using them to predict injury risk in humans.
The improved understanding of the BBB could also help further research into drug delivery of brain-specific medicines.
This study was funded by Wellcome Trust and Royal British Legion Centre for Blast Injury Studies at Imperial College London.
“Multiscale modeling of cerebrovascular injury reveals the role of vascular anatomy and parenchymal shear stresses” by Siamak Farajzadeh Khosroshahi et al., published 21 June 2021 in Scientific Reports.
Caroline Brogan, Imperial College London

