Early cognitive decline may result from a shift in the ratio of a protein sub-type in our brain cells triggering cell-loss. This new study, published in Scientific Reports, shows how this might be caused. The discovery provides a new therapeutic target to prevent the onset of neurodegenerative diseases including dementia and Alzheimer’s.
The study is the first to examine the effects on nerve activity of altering the balance of a key protein known as tau. Elevated amounts of the protein, which accumulates and forms tangles in the brain, is a hallmark of both dementia and Alzheimer’s. Although tau is well documented as one of the main damaging processes behind these diseases, until now its role in early-cognitive decline has not been well understood.
In normal human brain cells there are approximately equal amounts of the six subtypes of tau, comprising three grouped together as 4R-tau, and three as 3R-tau. Scientists believe when there is a shift in the ratio of 4R-tau subtypes, this increase has adverse effects resulting in early cognitive impairment, with the same effect also seen in aged animals.
To test this ratio shift theory, researchers from Bristol’s Schools of Physiology, Pharmacology and Neuroscience and Biochemistry, examined the 4R-tau subtypes in more detail, and the acute effects of shifting the balance of the different subtypes on brain cell nerve activity.
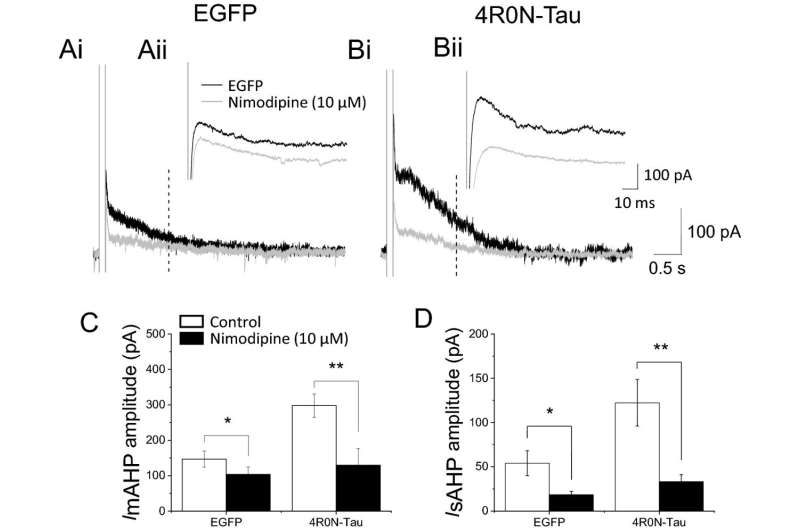
Using animal models, the team found that two 4R-tau subtypes increased the number of L-type calcium channels in brain cells, while the third did not. The effect of the other two 4R-tau subtypes was produced from a direct protein:protein interaction between tau and the calcium channel. This increase in the number of L-type calcium channels (which also occurs in aged animals) caused the brain cell’s activity to be dampened by an enhanced inhibitory response known as the slow afterhyperpolarization (slow AHP). The slow AHP is produced by calcium ions entering the brain cell through a protein ion channel known as the L-type calcium channel.
The team showed that the effect in the hippocampus resulted from more calcium entering the neurons through L-type calcium channels, which supports existing evidence that nerve death in dementia is caused by calcium overload.
Their discovery shows the changes that occur in a hippocampal nerve during early cognitive impairment are changes that occur before the patient is finally diagnosed with dementia.
Professor Neil Marrion, Professor of Neuroscience at Bristol’s School of Physiology, Pharmacology & Neuroscience, and the study’s lead author, said, “Our exciting findings suggest that preventing the direct interaction between 4R-tau subtypes and L-type calcium channels could be used as a therapeutic approach to prevent the onset of dementia.”
University of Bristol

