A rapid genetic testing model for patients with advanced prostate cancer can more quickly identify those with “actionable” gene variants eligible for newer targeted therapies, reports a clinical trial in The Journal of Urology.
“Oncologist-initiated germline genetic testing is a feasible approach to testing prostate cancer patients,” comments senior author Maria I. Carlo, MD, of Memorial Sloan Kettering Center, New York. “In our sample, nearly 10% of patients had a gene variant that made them eligible for effective new therapies, with faster results than traditional referral for genetic testing and counseling.”
Need for new models to meet demand for genetic testing of prostate cancer
Research has identified several pathogenic variants (PVs)—most commonly involving DNA-damage repair genes—that affect the behavior and clinical outcomes of advanced prostate cancer. Some of these PVs can be targeted by immunotherapy or other newer treatments, with the potential to improve tumor responses and patient survival, compared to standard treatments.
A key example is a class of medications called PARP inhibitors, which are effective in killing prostate cancer cells associated with mutations of the BRCA1 and BRCA2 genes—variants that are also linked to familial breast and ovarian cancer. PARP inhibitors have been approved for the treatment of prostate cancers when relevant PVs are present.
Availability of these and other new treatments has greatly increased the demand for genetic testing of advanced prostate cancers. However, traditional referral to a genetics clinic can take weeks or even months, with a further wait until results are available. “This model is unlikely to accommodate the up to 30,000 patients per year with new metastatic prostate cancer who may need time-sensitive genetic test results for therapeutic decision making,” Dr. Carlo and colleagues write,
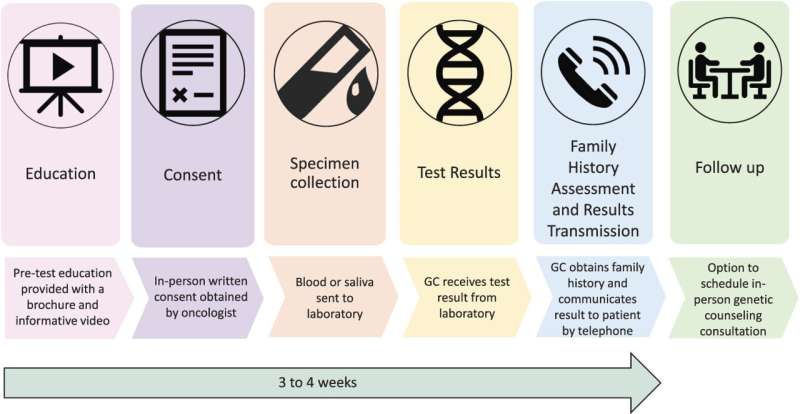
The researchers developed and evaluated a “mainstreaming” model to make timely genetic testing more readily available for patients with advanced prostate cancer. In this approach, oncologists identified patients appropriate for genetic testing at routine office visits, rather than going through the traditional referral process. A key first step was pre-testing education, including a brief, patient-friendly educational video.Play
00:00
07:58MuteSettingsPIPEnter fullscreen
PlayCredit: Wolters Kluwer Health
Nearly half of patients with PVs had ‘therapeutically actionable results’
After learning about genetic testing, 510 patients consented to the procedure, with samples collected for testing of 14 genes associated with hereditary prostate cancers. Follow-up was provided by a genetic counselor, who contacted the patients by telephone to obtain family history and discuss the results.
The streamlined genetic test model identified pathogenic gene variants in 51 patients, for a rate of 10.2%. Of these 51 patients, 47 had variants involving BRCA or other DDR genes. Sixty-one percent of patients had at least one with a history of breast cancer. Reflecting the demographics of the population served by the study centers, about one-third of the patients with PVs were of Ashkenazi Jewish ancestry: a group with elevated risk of hereditary prostate cancer.
Forty-seven patients had a “therapeutically actionable result,” involving a PV for which some form of approved or investigational targeted therapy is available. Overall, in 22 of 48 advanced prostate cancer patients who had a PV, the genetic findings led to discussions of a possible change in treatment.
In follow-up evaluations, patients were highly satisfied with the pre-test education process and with their decision to undergo genetic testing. Results were delivered to and discussed with patients a median of 20 days after sample collection.
“This study demonstrated the feasibility and clinical impact of utilizing an alternative model of genetic testing in individuals with advanced prostate cancer,” Dr. Carlo and co-authors conclude. They add: “This process and education material could serve as a model to other institutions experiencing a high volume of prostate cancer patients.”
Wolters Kluwer Health

